Pipeline
Building a portfolio to change post-surgical pain management forever
Allay’s first product candidates are designed to provide the weeks of pain management required for successful transition into post-surgical rehabilitation, reducing the need for constant re-dosing, clinical oversight and drawbacks of systemic analgesics such as opioids. Allay’s pipeline is built on its foundational platform technology that combines validated non-opioid analgesics and biopolymers for a new category of ultra-sustained therapeutics.

ATX-101: To Support Recuperation From
Total Knee Replacement Surgery
Allay’s first product candidates are designed to provide the weeks of pain management required for successful transition into post-surgical rehabilitation, reducing the need for constant re-dosing, clinical oversight and avoiding the drawbacks of systemic analgesics such as opioids.
Breakthrough pain associated with TKAs often results from significant gaps in pain relief from three days to approximately two weeks post-surgery. Allay believes patient preference for a non-opioid solution will drive demand to those clinicians offering innovative solutions designed to help patients recover faster and with less pain.
ATX-101 is an investigational product that has not been approved by the U.S. Food and Drug Administration
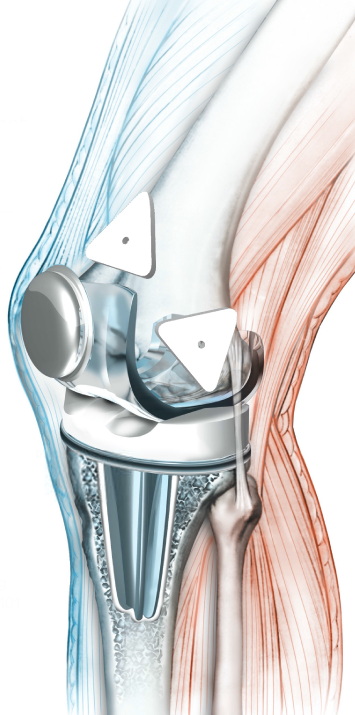
ATX-101 is a small product placed directly into the TKA surgical site in a simple, ‘place & go’ manner designed to add minimal extra complexity and time to the procedure. The local analgesic incorporated into ATX-101’s drug-biopolymer architecture is bupivacaine, an intracellular sodium ion channel blocker, which acts by inhibiting ion channels that span the membrane of sensory neurons. This inhibits the neuron’s ability to conduct signals to the spinal cord, including those from nociceptors, or pain receptors. Bupivacaine has historically been limited by its short duration of action – but now Allay’s technology may extend its proven capabilities by an order of magnitude.
ATX-101 incorporates the key features of Allay’s platform technology, including:
Non-opioid mechanism: ATX-101 incorporates bupivacaine, a proven local analgesic supported by decades of safety data and a high therapeutic index.
Selective local action: ATX-101 is only where it needs to be. It is designed to distribute only within the knee joint, minimizing exposure elsewhere in the body.
Simple administration: ATX-101 is implanted with a simple ‘place-and-go’ approach designed to add minimal extra complexity and time to the TKA procedure.
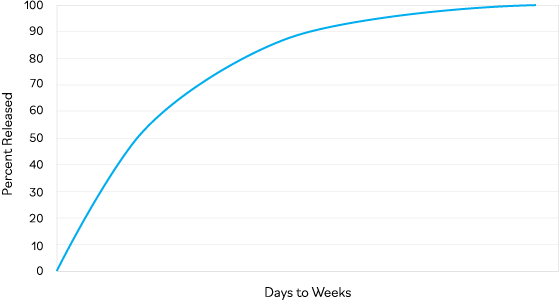
Extended and controlled release: ATX-101 is designed to continuously release declining amounts of analgesic, providing weeks of carefully calibrated pain relief to align with a patient’s natural recovery and pain experience.
Natural resolution: Over time, ATX-101 naturally disappears from the body.